Dan Summers
Research summary
Protein homeostasis in developing and diseased neurons
Background
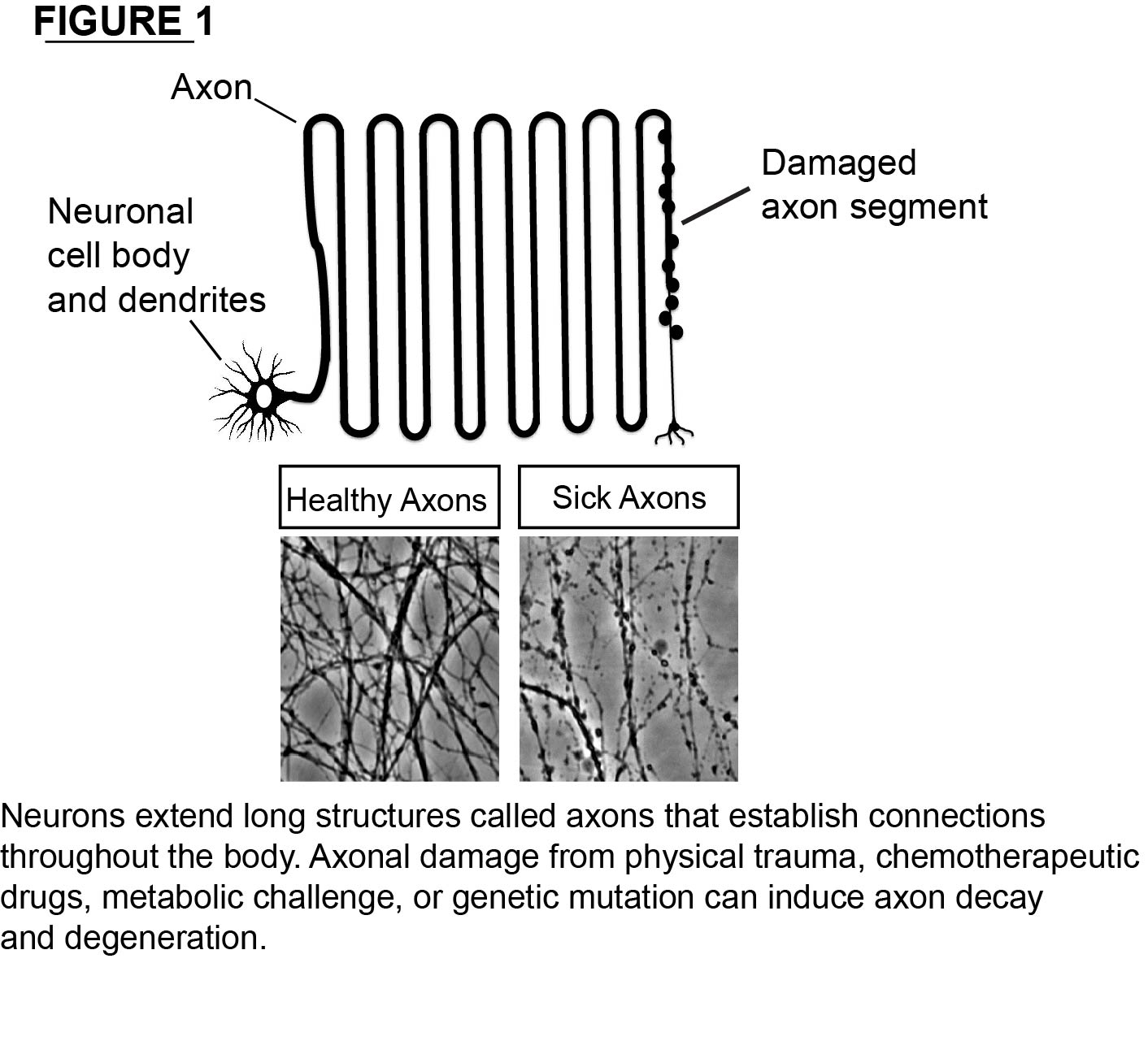
The nervous system controls every aspect of how we perceive and react to the world around us. To accomplish this incredible task, neurons extend long projections called axons that establish connections throughout the human body. Importantly, the neurons in our body are post-mitotic so axons must survive and function for our entire lifetime. Unfortunately, axon degeneration is a prominent event in many neurodegenerative disorders of the peripheral and central nervous systems (see Figure 1). Despite the essential role for axons in functional connectivity of the nervous system, treatments that protetct the axon compartment are limited.
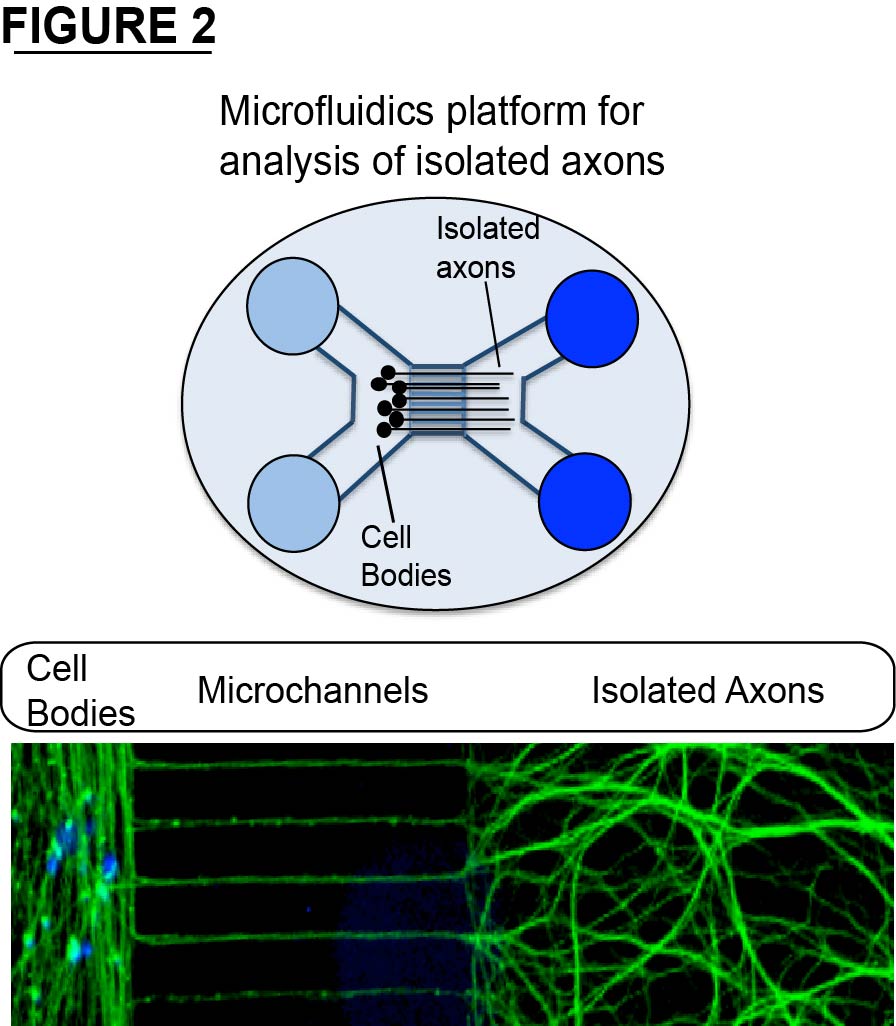
Axons can reach tremendous lengths (over 1 meter in humans) and carry out many basic biological activities in isolation from the neuronal cell body. For example, axons invest considerable energy toward sustaining local protein homeostasis (proteostasis). Defects in protein homeostasis underlie many neurological disorders including Alzheimer's disease, Parkinson's disease, and numerous peripheral neuropathies. In the Summers Lab, we investigate cellular pathways that regulate protein homeostasis in axon segments. We use a combination of biochemistry, live imaging, primary neuron models, and animal models to address this problem (see Figure 2 for examples). Below are examples of different questions we are addressing in the Summers Lab.
How do proteostasis networks regulate axon vulnerability or resistance to disease?
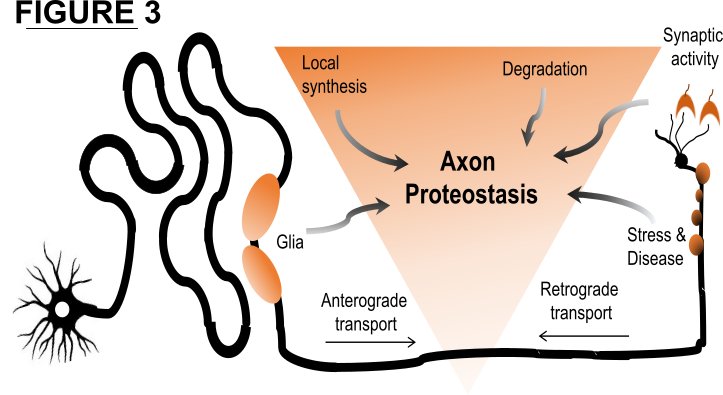
Axons are specialized cellular compartments that utilize many undiscovered strategies for regulating the biogenesis and disposal of axonal proteins. These proteostasis networks have important implications for neuronal function and survival. For example, axons require a constant supply of survival proteins and must efficiently balance protein transport and synthesis with regulated protein degradation (see Figure 3). Disturbing this balance can deplete axons of essential factors and provoke axon degeneration or enhance vulnerability to stress and dysfunction. Conversely, blocking specific protein degradation pathways can increase the abundance of these factors and promote axon survival. Therefore, we study the protein homeostasis of axon survival factors and what molecular pathways regulate this balance with the goal of boosting axon resistance to degeneration.
How does local protein synthesis affect axon biology and susceptibility to disease?
To circumvent transporting proteins across long distances, axons locally synthesize many proteins needed for function. Local synthesis is not a simple process and requires numerous auxiliary factors (ex. molecular chaperones) to facilitate the maturation of a new polypeptide into a functional protein. We are investigating the biogenesis of locally synthesized axonal proteins, how these proteins are properly folded, localized, and regulated in response to stress. We are also developing animal models to explore this question in vivo and in the context of chronic neurodegeneration.
How do stress response pathways intersect to elicit an adaptive (or maladaptive) cellular outcome?
Neurons possess multiple stress response pathways that sense changes in the external as well as internal cellular environment. The consequences of activating a stress response pathway are not always beneficial as over-stimulation can aggravate neuronal dysfunction. We are characterizing the spatial-temporal dynamics of stress response complexes (ex. MAP Kinases) in neurons and how they coordinately elicit local changes in axon compartments as well as transcriptional adaptations.
How does local environment influence neuron proteostasis?
Neurons do not exist in isolation. Rather, neurons interact with glia, immune cells, and other cell types in their environment. More and more, we are coming to appreciate the powerful influence these interactions have on neuron health and function. My lab investigates how non-cell autonomous signals from supporting glia and immune cells influence neuronal proteostasis. We suspect these pathways will have important implications for neuronal function and degeneration in disease.
Selected images
- Cell and developmental biology
- Neurobiology
