Jim (Jung-Ching) Lin
Research summary
Cardiac fevelopment and function; Roles of Xin repeat-containing proteins in cardiac development, function, and disease
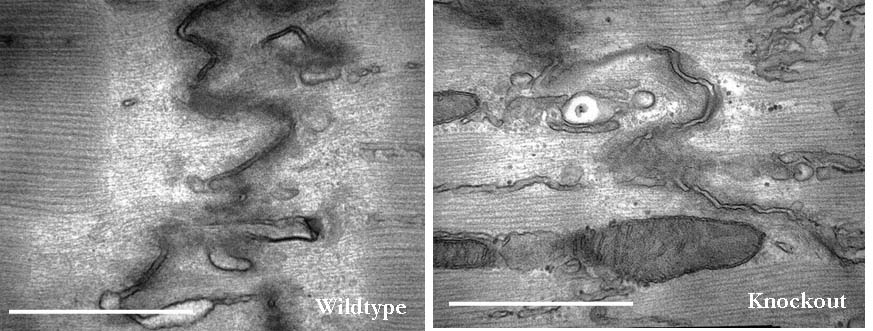
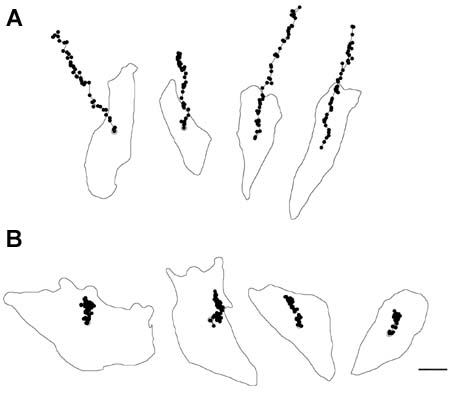
Since the discovery of Xin repeat-containing proteins from my laboratory in 1996, the importance of Xin proteins in cardiac development, function, and disease has been continuously implicated. Diverse vertebrates including mammals possess two paralogous genes, Xinα (or Xirp1) and Xinβ (or Xirp2). The majority of Xin proteins are localized to intercalated discs (ICDs) of the heart. Ablation of the mouse Xinα (mXinα) leads to progressive ICD-structural defects, cardiomyopathy, and conduction defects. In mXinα-deficient mice, the mouse Xinβ (mXinβ) is up-regulated at both message and protein levels. Complete loss of mXinβ results in the failure of forming mature ICDs and the mis-localization but no up-regulation of mXinα, suggesting a functional hierarchy between mXinα and mXinβ. The mXinβ-null mice show abnormal heart shape, ventricular septal defects, severe growth retardation and postnatal lethality. Our evolutionary studies reveal that emergency of Xin repeat-containing proteins coincides with the genesis of true chamber heart. This together with ICD-structural defects seen in adult mXinα-deficient hearts and with abnormal heart shape/ventricular septal defects detected in mXinβ-null hearts suggest that Xin proteins could play vital roles in heart chamber formation. Toward this end, we have used yeast two hybrid system, co-transfection and co-immnuoprecipitation to identify and characterize many mXin-interacting proteins. We have previously shown that mXinα interacts with β-catnin, p120-catenin, and actin filaments, and likely regulates the N-cadherin-mediated adhesion at ICD. The cadherin engagement is known to activate Rac1 through c-Src-PI3K-Vav2 (a guanine nucleotide exchange factor capable of binding to p120-catenin) pathway. Consistently, the loss of mXinβ fails to form ICD and down-regulates the activities of Rac1, IGF-1 receptor, Akt, Erk1/2, and cytokine receptor. We have further shown that mXinα interacts with Kv channel interacting protein 2 (KChIP2, an auxiliary subunit of Ito,f), filamin, cortactin, and Kcne2 (a β-subunit of Ik,slow1), and modulates the surface expressions of transient outward K+ channel (Ito,f) and the delayed outward K+ rectifier (Ik,slow1), both of which are critical components for defining action potential waveform. Thus, mXinα and mXinβ may act as scaffolding proteins to modulate various receptor/channel activities and signaling. In addition to characterizing more mXin-interacting proteins, we will use bioinformatics (biomart) analyses in collaboration with Dr. Albert Erives to systematically identify mXin co-originated/co-evolved partners that play roles in chamber formation and function. Interestingly, we have also found that the left atrial-pulmonary vein tissues prepared from mXinα-null hearts blunt isoproterenol-treated, pacing-induced atrial fibrillation, in contrast to tissues prepared from wild type hearts. The mXinα-deficient mice may provide a good opportunity to identify pathway involving in the pacing-induced atrial fibrillation. Studies with a second mXinβ allele mouse line with knock-in lacZ reporter have revealed that mXinβ is enriched in atria and conduction system, which may account for our preliminary observation of conduction defects in mXinβ-null mice. We will characterize molecular changes in cardiac conduction system and electrophysiological remodeling of mXinβ-null hearts.
Selected images