Steven Green
Research summary
Neurodegeneration, neural regeneration, and neuroimmune interactions in the mammalian cochlea
The Green Lab investigates neurodegeneration caused by trauma in the peripheral auditory system. The spiral ganglion of the cochlea experiences post-trauma neurodegeneration and neuroimmune interactions similar to those in the brain but is a simpler, more experimentally tractable, neural system.
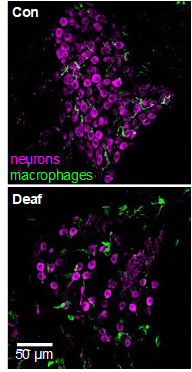
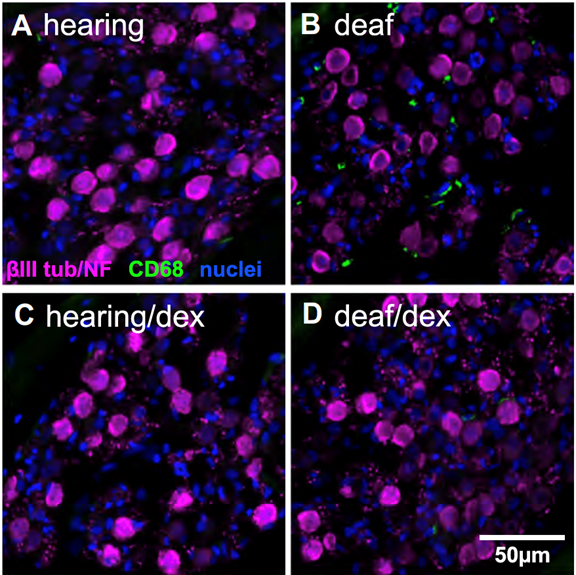
The spiral ganglion neurons (SGNs) connect the auditory sensory cells (“hair cells”) to the brain. The hair cells are susceptible to damage caused by loud noise, certain antibiotics and chemotherapeutics, infections, and genetic or congenital disorders. These can result in death of the hair cells and hearing loss, but the use of a cochlear implant, which electrically stimulates the SGNs directly, partially restores hearing. However, hair cell death can result in degeneration of the SGNs, potentially reducing the efficacy of cochlear implants. This death of SGNs consequent to hair cell loss has been extensively investigated using animal models in several laboratories, including our own and one area of research in our lab, begun in 1994, is focused on SGN degeneration and death. Our studies have shown that hair cell death is an indirect, rather than direct, cause of SGN death. Specifically, using gene expression profiling, confocal microscopy, and treatments with anti-inflammatory agents, we have shown that hair cell loss causes inflammatory and immune responses (Figure 1), and that it is these that cause SGN death (Figure 2) (Rahman et al., 2023). The immune response is complex involving both the innate and adaptive components. We are currently using transgenic rat and mouse models to identify those specific component(s) of the immune response responsible for SGN death.
A second issue under investigation in our lab is that of noise-induced cochlear synaptopathy (NICS). As noted above, loud noise will kill hair cells causing hearing loss. However, this is not the only hazard posed by noise. Moderate noise levels, that do not endanger hair cells, can destroy synaptic connections between the hair cells and the SGNs, reducing communication from the hair cells to the brain. This type of damage does not cause profound hearing loss but is thought to cause hearing impairments such as tinnitus (ringing in the ears) and difficulty in comprehending speech in noisy environments. Because these impairments are not generally detected by routine audiometry, the damage is referred to as “hidden hearing loss.” NICS can be caused by noise levels commonly encountered in occupational, recreational, or military environments. The impairments associated with NICS are the most common health-related complaint among military veterans.
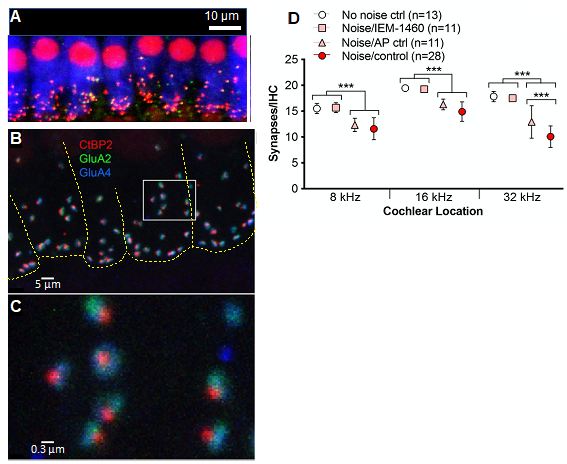
We have three projects relevant to NICS: First, in experiments using noise-exposed adult mice, we have identified the direct cause of the damage to cochlear synapses. Noise causes increased release of the neurotransmitter glutamate from hair cells, which overstimulates the glutamate receptors on the SGNs. This results in a pathological influx of ions, especially calcium ion, Ca2+, through a subtype of glutamate receptors, which lack the GluA2 receptor subunit, termed Ca2+-permeable AMPA receptors (CP-AMPARs). A selective blocker of CP-AMPARs prevents synaptopathy without affecting hearing because other subtypes of glutamate receptors remain unblocked (Hu et al., 2020). (Watch a YouTube video summarizing some of these results) These studies also showed that each postsynaptic density has subdomains – which we termed “nanodomains” – that are either enriched or deficient in GluA2 and, thus, relatively Ca2+-impermeable or Ca2+-permeable (Figure 3). This explains why Ca2+ influx can be blocked with CP-AMPAR blockers, preventing synaptopathy, but sufficient receptors remain unblocked to allow normal hearing. With our colleagues at Washington University in St. Louis, we are currently working to develop novel CP-AMPAR blockers that function at lower dosages and with greater specificity, for use by people in noisy environments.
Second, we are investigating strategies for promoting regeneration of cochlear synapses that have been destroyed by NICS. Our earlier work focused on peptide neurotrophic factors (Wang & Green, 2011). Other labs have also investigated the use of these and other peptides. While these approaches succeeded in restoring synapses, the peptides had to be introduced intracochlearly, requiring invasive surgery, an approach unsuitable for use in humans. We therefore have more recently focused on approaches using small molecules that be delivered systemically by injection or orally. We have now identified at least one such small-molecule compound that has successfully promoted synapse regeneration in vivo in noise-exposed mice.
Third, in the course of these studies, we noticed that female mice recovered from NICS much better than did male mice, showing reduced hearing impairment and a greater number of surviving synapses. Interestingly, the recovery varied over the course of the estrus cycle, in parallel with serum levels of progesterone. We are now investigating the mechanism by which progesterone promotes synapse regeneration.
Our research is supported by grants from the NIH/NIDCD and from the Hearing Restoration Research Program of the Department of Defense.
Cited references
Rahman MT, Bailey EM, Gansemer BM, Pieper AA, Manak JR, Green SH. Anti-inflammatory therapy protects spiral ganglion neurons after aminoglycoside antibiotic-induced hair cell loss. Neurotherapeutics. 20(2):578-601, 2023.
Hu N, Rutherford MA, Green SH. Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors. Proc Natl Acad Sci U S A. 117(7):3828-3838, 2020.
Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 31(21):7938-49, 2011.
Selected images
- Cell and developmental biology
- Neurobiology
